Runcaciguat
Product Detail
Product Tags
| Pack Size | Availability | Price (USD) |
| 10mg | In Stock | 390 |
| 100mg | In Stock | 850 |
| 1g | In Stock | 3560 |
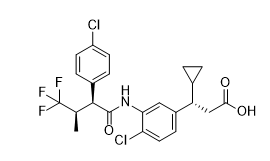
Chemical Name:
(3S)-3-{4-chloro-3-[(2S,3R)-2-(4-chlorophenyl)-4,4,4-trifluoro-3-methylbutanamido]phenyl}-3-cyclopropylpropanoic acid
SMILES Code:
O=C(O)C[C@H](C1=CC=C(Cl)C(NC([C@H](C2=CC=C(Cl)C=C2)[C@@H](C)C(F)(F)F)=O)=C1)C3CC3
InChi Code:
InChI=1S/C23H22Cl2F3NO3/c1-12(23(26,27)28)21(14-4-7-16(24)8-5-14)22(32)29-19-10-15(6-9-18(19)25)17(11-20(30)31)13-2-3-13/h4-10,12-13,17,21H,2-3,11H2,1H3,(H,29,32)(H,30,31)/t12-,17+,21+/m1/s1
InChi Key:
NCRMKIWHFXSBGZ-CNBXIYLPSA-N
Keyword:
1402936-61-1;CAS:1402936-61-1;CAS:1402936-61-1;Runcaciguat; BAY 1101042; BAY-1101042 BAY1101042
Solubility: Soluble in DMSO
Storage: Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years)
Description:
Runcaciguat, also known as BAY 1101042, is a novel, potent, and orally active sGC activator. Runcaciguat activated the sGC reporter cell line with an EC50 value of 11.2 ± 1.0 nM. Pretreatment of the sGC reporter cell line with 30 μM ODQ for 3 h resulted in an increased potency of runcaciguat (EC50 of 2.1 ± 0.07 nM), and treatment of the reporter cells with runcaciguat in combination with the NO donor S-nitroso-N-acetylpenicillamine (SNAP) (10 and 100 nM) showed additive effects. Treatment with runcaciguat resulted in maximal luminescence signals in the range of 50–60% in comparison to the sGC activator cinaciguat. Given the broad impact of oxidative stress in cardiovascular and cardiorenal diseases, runcaciguat might become a new treatment modality for a broad variety of diseases in these indication space but also beyond.
Target: Soluble guanylate cyclase promoter