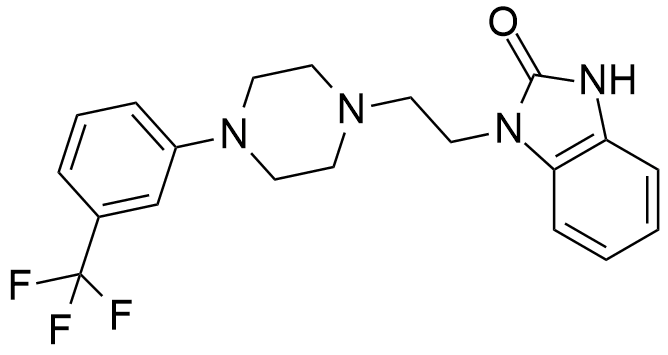
Flibanserin
Product Detail
Product Tags
| Pack Size | Availability | Price (USD) |
| 1kg | In Stock | 1850 |
| More Sizes | Get Quotes | Get Quotes |
Chemical Name:
1-(2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethyl)-1H-benzo[d]imidazol-2(3H)-one hydrochloride
SMILES Code:
O=C1NC2=CC=CC=C2N1CCN3CCN(C4=CC=CC(C(F)(F)F)=C4)CC3
InChi Code:
InChI=1S/C20H21F3N4O/c21-20(22,23)15-4-3-5-16(14-15)26-11-8-25(9-12-26)10-13-27-18-7-2-1-6-17(18)24-19(27)28/h1-7,14H,8-13H2,(H,24,28)
InChi Key:
PPRRDFIXUUSXRA-UHFFFAOYSA-N
Keyword:
Flibanserin, 167933-07-5
Solubility: Soluble in DMSO
Storage: 0 - 4°C for short term (days to weeks), or -20°C for long term (months).
Description:
Flibanserin is a full agonist of the 5-HT1A receptor (Ki = 1 nM) and, with lower affinity, as an antagonist of the 5-HT2A receptor (Ki = 49 nM) and antagonist or very weak partial agonist of the D4 receptor (Ki = 4–24 nM). Despite the much greater affinity of flibanserin for the 5-HT1A receptor, and for reasons that are unknown, flibanserin occupies the 5-HT1A and 5-HT2A receptors in vivo with similar percentages. Flibanserin also has low affinity for the 5-HT2B receptor (Ki = 89.3 nM) and the 5-HT2C receptor (Ki = 88.3 nM), both of which it behaves as an antagonist of. Flibanserin preferentially activates 5-HT1A receptors in the prefrontal cortex, demonstrating regional selectivity, and has been found to increase dopamine and norepinephrine levels and decrease serotonin levels in the rat prefrontal cortex, actions that were determined to be mediated by activation of the 5-HT1A receptor.[12] As such, flibanserin has been described as a norepinephrine-dopamine disinhibitor (NDDI). Flibanserin was approved in August 2015 for the treatment of pre-menopausal women with hypoactive sexual desire disorder (HSDD).
Target: 5HT